| Biological Function | ||||||
 |
||||||
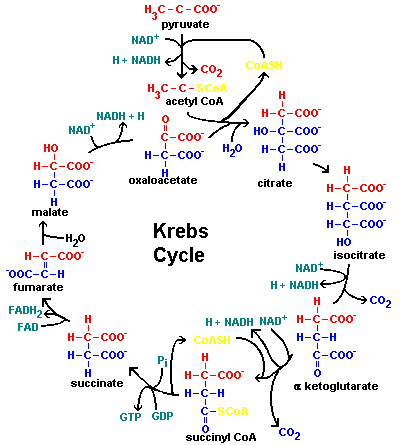
| Fumarase is an enzyme in the citric acid cycle, the glyozylate cycle, and the urea cycle which catalyzes the addition of water to the double bond of fumarate to form L-malate. The enzyme is stereospecific which works only on the trans isomer. The mechanism is as follows: Fumarate + H2O <=> L- Malate
In the metabolic citric acid cycle, or Krebs cycle, fumarase elicits this reaction in both the forward and reverse reactions. Fumarase can be divided into class I and class II types. Both these classes are found in Escherichia coli. Class I fumarases are iron dependent, 4Fe-4S cluster containting, dimeric proteins which weigh in at 120 kDa. Two examples of the class I fumarase are fumarase A and fumarase B. These two are approximately 90% sequesce specific. Fumarase C, also known as Fumarate Hydratase, is a member of the class II enzymes. Fumarase C is a tetramer. Each polypeptide chain contains 467 amino acids. None of these chains require any metal ions for the initiation of the enzyme. No unstable 4Fe-4S clusters are found in fumarase C. With both of these classes of enzymes present, the host can adapt to unfavorable oxygen states by inducing fumarase C in it's O2 stable form. |
||||||