| Mechanism of Succinyl-CoA Synthetase | |||||||||||||||||||
|
|
|||||||||||||||||||
| Mechanism of Succinyl-CoA Synthetase | Model of Important Sites for the Mechanism | ||||||||||||||||||
| The Histidine 246 catalytic sites with the associated phosphate groups and CoA groups. | |||||||||||||||||||
 |
|||||||||||||||||||
| The Histidine 142 facilitative catalytic residues in relation to the items in the previous button. | |||||||||||||||||||
| It is not recommended that you rotate this diagram after you have pushed any buttons because the zoom set for the buttons will cause you to lose sight of what is there. | |||||||||||||||||||
| Also, make sure that the buttons are pushed in order or weird things will happen. | |||||||||||||||||||
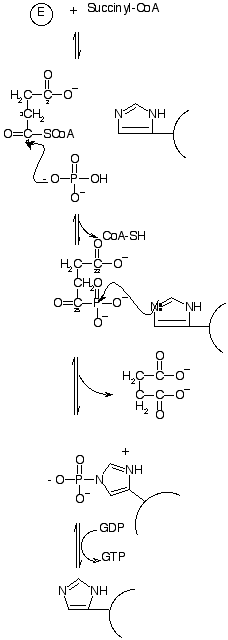
| As mentioned previously, there are three steps in the mechanism of the conversion of succinyl-CoA to succinate by SCS. These reactions were shown on the introduction page and the more detailed diagram showing the actual mechanism is shown to the left. On the interactive diagram above, I have shown the histidine 246 molecules that compose the catalytic sites of SCS and also the additional facilitative catalytic site of histidine 142. Also the Phosphate groups associated with histidine 246 and the CoA molecules that bind the enzyme are shown. The equilibrium constant for the reaction is about 1, so the amount of nucleotide present tends to control which direction will proceed. If ADP is present in higher concentrations than ATP, then the reaction will proceed in the forward direction and change succinyl-CoA into succinate.
There has been evidence found for allosteric regulation of SCS. There is a high-affinity GDP-binding site that has been found to regulate the enzyme allosterically. This allosteric regulation actually increases the rate of SCS phosphorylation. Another unusual characteristic of the SCS enzyme is one of its catalytic properties which is called substrate synergism. What this means is that the presence of a substrate for one partial reaction stimulates another partial reaction. In E. coli, since there are two catalytic sites, there is alternating catalytic co-operativity. In other words, when a substrate binds one of the catalytic sites, it facilitates the catalytic events at the other catalytic site. This mechanism of catalysis only holds for E. coli, though. In order for substrate synergism to work in mammals, there needs to be a slightly different mechanism since there is only one catalytic site. In this different mechanism, binding of a substrate to the catalytic site enhances another partial reaction to occur at that same site. In conclusion, the information being obtained about SCS in mammalian systems is still in the relatively early stages of research. A structure for GTP-specific SCS found in pig hearts was just solved in June of 2000. Because of this, researchers are just starting to find out more about how this enzyme functions in mammalian systems and also figuring out how exactly the catalytic mechanism is able to function in the unusual way it does. In other words, there is still much to be learned about the way it functions in the mammalian system and information can still be perfected in a bacterial system, also. |
|||||||||||||||||||
| Home Page | Introduction | Structure | Mechanism | References | |||||||||||||||