- It requires 26.3 mL of 0.10 M KOH to titrate a 35.6 mL sample of acetic acid (CH3COOH). Calculation the initial pH of the acetic acid solution.
- It requires 45.0 mL of 0.20 M HCl to titrate 25.0 mL of a Ca(OH)2 solution of unknown concentration. What is the Ca(OH)2 concentration?
- The balanced chemical equation for this reaction is
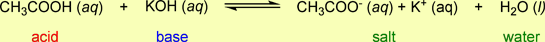
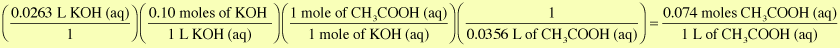
The initial concentration of the acetic acid is 0.074 M.
- This problem presents a couple of new twists to the titration problem. The first is, that in this case a base is being titrated with an acid instead of the other way around, so the unknown is the base concentration. The other twist is that the acid reacts with the base in a 2 to 1 ratio, instead of a 1 to 1 ratio. This is seen in the balanced chemical equation for this reaction:
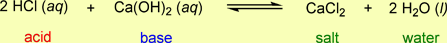
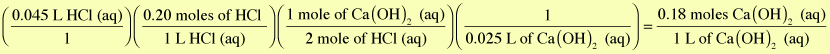
The initial concentration of the Ca(OH)2 is 0.18 M.