Zeolites and Catalysts: A Physical Chemistry perspective
By Adam Harder
If a zeolite
is strongly heated the absorbed water escapes just as if the stone is
boiling. Hence the term zeolite, which
is formed from Greek words meaning boiling stone. Zeolites are crystalline, hydrated aluminosilicates with a
framework structure. Their three-dimensional polyanionic networks are
constructed of SiO2 and AlO4 tetrahedra linked through
oxygen atoms. Depending on the
structure type, they have regular voids containing cations and water molecules
which are mobile and can be exchanged[1].
This ability to absorb and exchange ions and other molecules is what
makes zeolites such a big part of the chemical industry. The purpose of this paper is to briefly
summarize the chemistry and applications of zeolites used in industry today.
The
structure of zeolite crystals can be represented as a plurality of tetrahedra,
each of which consists of four oxygen atoms surrounding a smaller silicon or
aluminum atom. Each oxygen atom of a
tetrahedron can combine with another silicon or aluminum atom, which, in turn
forms a tetrahedron with four oxygen atoms.
Thus a solid lattice is built up from multiple tetrahedra[1]. The (-Si-O-Al-) linkages form surface pores
of uniform diameter and enclose regular internal cavities and channels of
discrete sizes and shapes, depending on the chemical composition and crystal
structure of the specific zeolite involved.
The enclosed cavities contain both metal cations and water
molecules. The cations are loosely
bound to the lattice and thus can engage in ion exchange. The water molecules can also be reversibly
driven off in most zeolites. The
regular nature of the pores and their apertures enables zeolites to function as
molecular sieves. This is the
outstanding property of zeolites that gives them their value as selective
adsorbents for separating substances and as shape-selective catalysts. Depending on the zeolite type and its pore
system, molecules can enter into the cavity system or be excluded from it. Most of the chemical and physical properties
of the zeolites, and hence their areas of use, are essentially determined by
the aluminum content of their frameworks.
In the literature, this is usually expressed by the Si/Al or SiO2/Al2O3
ratio. The surface selectivity of the
zeolites as adsorbents depends on this ratio. Aluminum-rich zeolites adsorb
strongly polar molecules, while increasing the silicon content leads to
increasingly hydrophobic character[2].
Many
synthetic zeolites also occur naturally as minerals. However, zeolites only became of industrial importance in the
1950’s, when synthetic examples became available on an industrial scale. When talking about the industrial use of
zeolites it is useful to break them down into three basic types: type A, type
X, and type Y. Typical chemical
compositions of these types are shown below.
Type A Na12(AlO2)12(SiO2)12
· 27H2O
Type
X Na86(AlO2)86(SiO2)106
· 264H2O
Type Y Na66(AlO2)56(SiO2)136
· 250H2O
_______________
[3]
The zeolite’s
pore size and shape determine the size of the molecules that can enter the
zeolite interior. Type A zeolites have
a pore diameter such that it is an excellent exchanger of calcium ions and are
consequently used as detergent builders.
Types X and Y zeolites have larger pore sizes than type A. In addition to the effect of the surface
pores in restricting the size and shape of molecules entering and leaving the
zeolite, the size and shape of the internal cavities determine which transition
states are allowed in a reaction and which products can be formed[2]. Consequently the type X and Y zeolites are
used as catalysts in the catalytic cracking of petroleum, the largest zeolite
catalyst market. Today the type Y
zeolites have taken over this market due to their higher thermal stability
caused by their relatively higher silica/alumina ratio.
Although
there are many processes for zeolite synthesis, most commercial zeolite
production can be categorized into two main types of processes:
¨The
formation and crystallization of the zeolite from basic raw materials, via sols
or an aluminosilicate hydrogel.
¨The
crystallization of the zeolite in situ from calcined kaolin clay.
Hydrogel/Sol Processes
Typical sodium zeolites are formed by the crystallization
of sodium aluminosilicate prepared from pure sodium aluminate, sodium silicate,
and sodium hydroxide solutions.
NaOH +
NaAl(OH)4 + Na2SiO3 + H2O
¯
[(Na)a(AlO2)b(SiO2)c
· NaOH · H2O]gel
¯
(Na)m[(AlO2)m(SiO2)n]
· pH2O + mother
liquor
Factors affecting the type and
structure of the zeolite formed include the crystallization temperature and
length of time that the gel is maintained at that temperature, the
silica/alumina ratio of the reaction mixture, and the size and type of cation
present[3].
Kaolin
Conversion Process
Kaolin clay can also be used as a source of alumina and
silica for zeolite synthesis. For
example, meta-kaolin is produced by calcining kaolin at 500-600°C. Zeolite A is then formed by the reaction of
meta-kaolin with aqueous sodium hydroxide.
A source of additional silica, such as sodium silicate, is required for
production of type X and Y zeolites with this method[3].
The applications of synthetic zeolites then, can be broken
down into three major categories: zeolites as detergent builders, zeolites used
for adsorption and separations, and zeolites as catalysts. For the purposes of this paper I will focus
on the use of zeolites as catalysts.
The use of zeolites as heterogeneous catalysts is most
important in there use in oil-refining processes. The most important is fluid catalytic cracking (FCC), which
converts vacuum distillates and residues into gaseous alkenes, gasolines, and
diesel fuel. Type Y zeolites have been
used as active components in the FCC process since 1964. These materials constitute 5-40% of the
catalyst. Zeolite catalysts have higher
activity and give higher gasoline yields and less coke formation than the
amorphous alumina-silica and high-alumina catalysts formerly used. The presence of sodium ions in the zeolite
would deactivate the catalyst, so most of the sodium is removed by exchange
with ammonium and/or rare earth cations.
These catalysts can then be calcined to drive off ammonia, producing a
hydrogen ion-exchanged zeolite with a smaller unit cell volume than
conventional type Y zeolites. The rare
earth elements provide stability and high catalytic efficiency. The framework structure of these zeolites
creates intracrystalline pores called supercages, each with a diameter of about
1.2 nm. The pore structure is three
dimensional, and the supercages are connected by apertures with diameters of
about 0.74 nm[4]. These cages and
apertures allow a zeolite catalyst to be size selective, so it can “choose”
which sized molecules it wants to catalyze. Some rather large molecules can fit
through these apertures and undergo catalytic reaction in the cages.
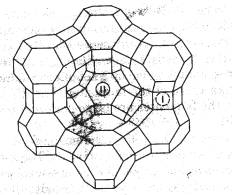
-Figure 1: Diagram of a
Zeolite Supercage[4].
The zeolite
frame is made up of SiO4 tetrahedra, which are neutral, and AlO4
tetrahedra, which have a charge of –1.
The charge of the AlO4 tetrahedra is balanced by the charges
of additional cations that exist at various crystallographically defined
positions within the zeolite. Zeolites
are thus ion exchangers, and the cations may be catalytically active. Analyzing
catalytic activity requires the computation of factors controlling reactions at
the catalytic sites in the zeolite. The electronic properties of the system are
thus critical.
Zeolite catalysts effect reactions in a number of
ways. For reactions with big activation
energies, such as in the catalytic cracking of petroleum, you would normally
need to add a large amount of energy to make the reaction proceed at any
reasonable rate. Introducing a catalyst
to the same reaction reduces the activation energy and allows the reaction to
go at a much faster rate without the need for the addition of extensive amounts
of energy. For then a higher proportion
of molecules are able to pass over the activation barrier.
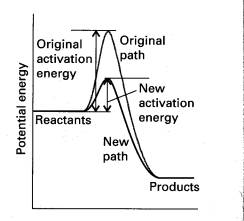
-Figure 2:
Potential Energy vs. Time[5].
Although the new route is faster, the
initial reactants and final products are the same. We know that G is a state function, so ΔGrӨ has the same value however the
reaction is brought about. Therefore,
an alternative pathway between reactants and products leaves ΔGrӨ, and
therefore K, unchanged[5]. That is the
presence of a zeolite catalyst does not change the equilibrium constant of the
reaction.
Overall, zeolites are very useful in
the process of separating petroleum into its different C fractions. They are size selective catalysts, which
gives them the advantage of being able to choose which C fractions to
separate. By changing the cations
present in their structure zeolites can be used to catalyze many other
reactions as well.
Bibliography
[1] L.W. Cod,
K. Dijkhoff, C.J. van Oss, H.G. Roebersen, E.G. Stanford (eds.): Chemical Technology: An Encyclopedic
Treatment, Vol 1, Barnes & Noble Inc., New York (1968) 136-137
[2] E. Roland, P. Kleinschmit, A.G. Degussa, Z.N.
Wolfgang, et al, “Zeolites,” Ullmann’s
Encyclopedia of Industrial Chemistry, Vol A28, VCH (1996) 475-500
[3] M. Smart,
T Esker, A. Leder, K. Sakota, “CEH Marketing Report: Zeolites,” Chemical Economics Handbook, SRI
International (1999) 599.1000A-599.1002U
[4] J. I.
Kroschwitz, M. Howe-Grant, et al, Encyclopedia of Chemical Technology: 4th
Ed, Vol 5, John Wiley and Sons Inc., (1993) 358-445
[5] P. Atkins,
The Elements of Physical Chemistry: with Applications in Biology, 3rd
Ed, W. H. Freeman and Co., New York, (2000) 158-238