For molecular substances, there are basically three states of matter, solids (s), liquids (l) and gases (g). Each of these states can be easily distinguished by their physical properties. In Section 5.1 of Raymond, water is used as example substance. Typically, a substance is converted from one state to another by adding heat; adding heat increases the kinetic energy (motion) of the molecules. Anyone who has experienced a winter in Wisconsin knows that the weather is markedly different depending on which side of the freezing point of water the temperature is at. For example, above the freezing point precipitation falls as a liquid rain, soaks into the ground or runs off into the gutters. Soon after the sun comes out, nearly all evidence of the rainfall is gone. Below the freezing point the situation is quite different. Precipitation falls as solid snow and collects on the ground. Even after the sun comes out, it can remain there for months, until spring arrives and the temperatures once again rise above the freezing point.
- Solids: As a solid, molecular compounds have a defined shape and volume that does not flow. This is why the snow remains where it falls.
- Liquid: As a liquid, molecular compounds have a defined volume but not a defined shape. They flow to adopt the shape of the container that holds them.
- Gases: As a gas, molecular compounds have neither a defined volume nor shape. Like liquids they flow to adopt the shape of the container, but unlike liquids, they also expand to fill the volume of the container that holds them.
No matter what state a molecular compound is in, the composition of the molecules that make up that substance remains the same. For example, the molecules of water are made up of H2O, regardless of whether it is a solid (ice), or a liquid (water), or a gas (water vapor). What changes when heat is added are the strength and numbers of interactions between the water molecules.
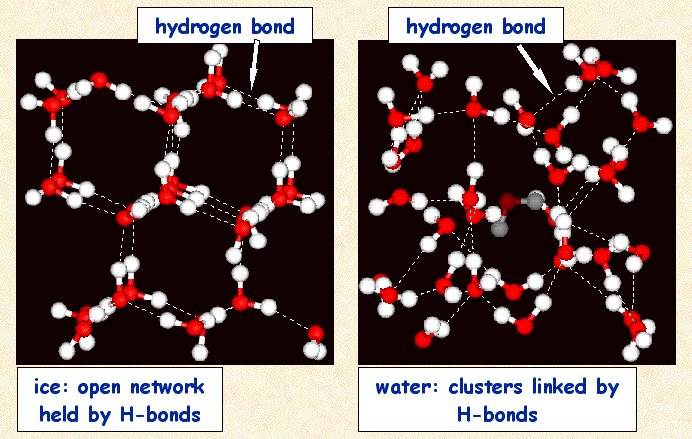
For the substance water, the primary noncovalent interaction that attracts one water molecule to another is the hydrogen bond. In the solid phases, the hydrogen bonds are numerous enough and strong enough to prevent the water molecules from flowing past one another. In the liquid phase, enough of these interactions are disrupted that the molecules can flow past one another, but enough of these interactions remain so that the water molecules are still held close to one another. In the gas phase, all of the interactions are broken and the water molecules are free to wander off independently of one another. Figure 1 illustrates the difference in structures between liquid water and ice:
|
Figure 1: The structures of liquid water and ice. For both, the water molecules are held close to one another by hydrogen bonds. In ice, the molecules are locked in position and unable to flow past one another, whereas in liquid water the molecules are able to flow past one another. |
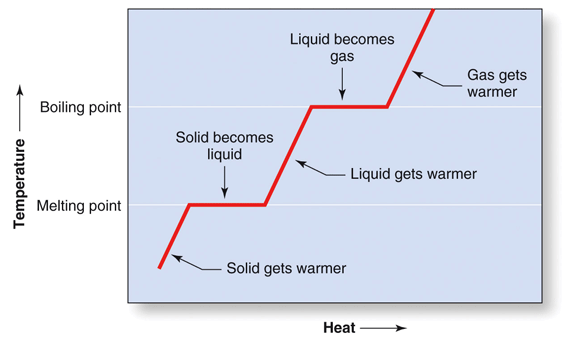
Heat is measure of the kinetic energy a substance contains. The more heat that is added to a substance, the more the molecules in that substance will move around and the hotter the substance will become. When heat is added to a solid, the temperature of the solid increases until enough energy is added to break the bonds that are holding the molecules in the fixed positions within the solid. At that point, adding more heat will no longer raise the temperature, but instead will be used disrupt intermolecular interactions and covert the solid to a liquid. Once all of the solid is converted to a liquid, the added heat will again cause the molecules to move around faster and the temperature of the liquid will rise. When enough heat has been added to the liquid to break the bonds that hold it in the liquid state, the temperature will again stop rising and instead will be used to disrupt the remaining interactions and convert the liquid to a gas. Once all of the liquid is converted to gas, the added heat will once again cause the molecules to move around faster and the temperature of the gas will rise. This behavior is illustrated in Figure 2:
|
|
Figure 3 below is an animation which illustrates the effect of adding heat to water, as it traces the curve shown in Figure 2, starting with ice and moving through the two phase transitions to liquid water and then to water vapor.
Phases Of Water
 |