Spontaneous changes are ones in which the free energy of a system decreases. Heat energy is also called enthalpy. When heat is released, the change in the enthalpy for the system that is releasing the heat decreases, whereas when heat is absorbed, the change in the enthalpy increases. While a decrease in the enthalpy makes a process more spontaneous (favorable), the change in enthalpy alone cannot be used to predict whether an overall change is spontaneous. There is another factor that must be considered and that is the entropy. Entropy is a measure of disorder; when a system become more disordered, the change in entropy is positive. When a change entropy is positive, it makes the change more spontaneous (favorable). Nature loves disorder. But like enthalpy, changes in entropy alone cannot be used to predict whether an overall change is spontaneous. For that you need to determine the change in the free energy. The free energy change combines the enthalpy change and the entropy change together, along with the temperature, to produce a quantity that can be used to determine if a process is spontaneous or not. This is summarized in the following equation:
![]()
where ![]() is the change in the free energy,
is the change in the free energy, ![]() is the change in the enthalpy,
is the change in the enthalpy, ![]() is the change in the entropy, and T is the absolute temperature in Kelvin. The following tables lay out the conditions for when a process is spontaneous and when it is not.
is the change in the entropy, and T is the absolute temperature in Kelvin. The following tables lay out the conditions for when a process is spontaneous and when it is not.
| Process is | Conditions | |
| + | nonspontaneous | always |
| – | spontaneous | always |
| Process is | Conditions | ||
| + | – | nonspontaneous | always |
| – | + | spontaneous | always |
| – | – | nonspontaneous | | |
| but can be made spontaneous by |
Lowering T | ||
| + | + | nonspontaneous | | |
| but can be made spontaneous by |
Raising T |
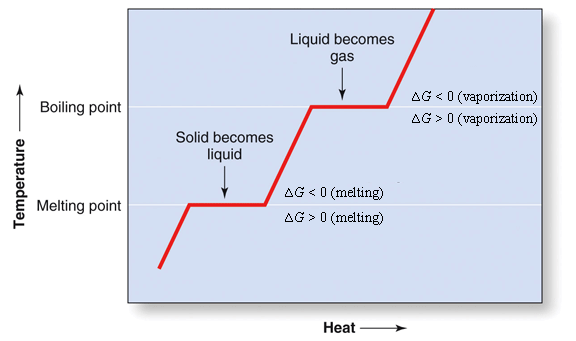
The "| |" brackets mean "the absolute value of". A good example of the last situation where both ![]() and
and ![]() are positive, is the heating of solid water (ice) to convert it first to a liquid and then to a gas. The
are positive, is the heating of solid water (ice) to convert it first to a liquid and then to a gas. The ![]() is positive because heat is being absorbed. The
is positive because heat is being absorbed. The ![]() is positive because the water becomes more disorder as it change from a solid to a liquid, and from a liquid to a gas (See Figure 1)
is positive because the water becomes more disorder as it change from a solid to a liquid, and from a liquid to a gas (See Figure 1)
|
|