Oxidation and reduction reactions involve transferring electrons from one atom or molecule to another atom or molecule. Oxidation occurs when electrons are lost, while reduction occurs when electrons are gained. The two processes are linked, whenever one substance is oxidized another has to be reduced.
There are different extents to which oxidation can occur. When metals react with nonmetals the metals tend to transfer their valence electrons completely to the nonmetal. As a consequence, the metals form positive ions while the nonmetals form negative ions. An example is the reaction of sodium metal with chlorine gas:
![]()
The sodium ions (Na+) and chloride ions (Cl-) combine to form solid sodium chloride, which is an ionic compound. In this reaction the sodium atoms each lose an electron and are therefore oxidized to sodium ions. The chlorine atoms each gain an electron and are therefore are reduced to a chloride ions. Another way to look at this is to say that the chlorine is the oxidizing agent because it takes the electrons away from sodium, while sodium is the reducing agent because it gives electrons to the chlorine atoms.
When nonmetals react with other nonmetals they tend to form covalent bonds, in which the valence electrons are shared instead of being transfered completely from one reactant to another. This is typical of the reactions that we seen in organic and biological chemistry. However, even though the valence electrons are being shared in these types of reactions, they often are not shared equally among the atoms that form the covalent bonds. Electronegative elements, such as oxygen and nitrogen, will tend to hold onto the valence electrons in a covalent bond a greater percentage of the time than the lesser electronegative elements, such as hydrogen. Even though electrons are not being transferred completely, we still consider a partial transfer of electrons from a less electronegative atom to a more electronegative atom as a form of oxidation and reduction. One way to recognize when this kind of oxidation and reduction occurs is to compare the oxidation numbers for all of the elements in the reactants and products of a reaction. This is something that you probably encountered in your general chemistry course. Another way to recognize when oxidation and reduction occur when covalent bonds are fomred is to apply the simple rules that are found on p.185 of Raymond:
An atom in a molecule is oxidized if it
- gains covalent bonds with oxygen
- loses covalent bonds with hydrogen
An atom in a molecule is reduced if it
- loses covalent bonds with oxygen
- gains covalent bond with hydrogen
The combustion reaction provides a good example. Combustion is a reaction with oxygen that forms carbon dioxide and water. The balanced chemical equation for the combustion of methane is
![]()
In this reaction, the carbon atom in methane gains 4 covalent bonds with oxygen (In CO2 the carbon forms double bonds to both oxygen atoms, O=C=O.) It also loses 4 covalent bonds with hydrogen. By either definition, the carbon has been oxidized. This is more easily seen using skeletal structures for the reactants and products:
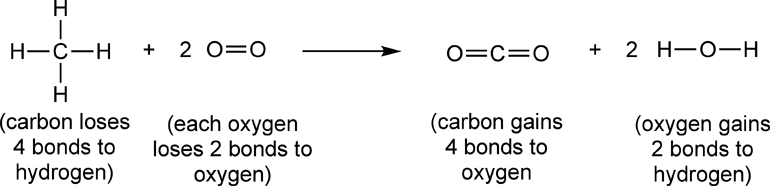
Also in this reaction the oxygen atoms in O2 lose covalent bonds with oxygen and gain covalent bonds with hydrogen, therefore, the oxygen atoms in O2 are reduced when they are converted to H2O.
Hydrogenation reactions are another example of oxidation/reduction reactions. The hydrogenation of ethene by H2 gas provides an example:
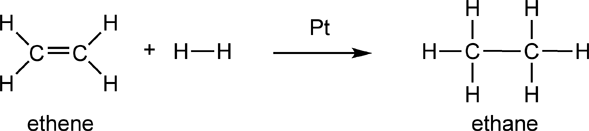
In this reaction, the two carbon atoms in ethene each gain covalent bonds to hydrogen, therefore they are being reduced. The hydrogen atoms in H2 each lose bonds to hydrogen, therefore they are being oxidized. The “Pt” above the reaction arrow stands for platinum, which helps catalyze this reaction. It is placed above the arrow to indicate that it is neither a reactant or product in this reaction. This same reaction is shown in p.187 in Raymond, but there they place the H2 above the arrow. I find this a confusing way to write the reaction equation since now the reaction equation is no longer balanced.
The reduction of a double bond with H2 in the presence of a Pt catalyst is a reaction that is carried out in the lab, however, biological system have a different way of reducing double bonds. They use large organic molecules, such as nicotinamide-adenine dinucleotide (abbreviated NADH + H+). On p.187 there is an example of a reduction of a carbon-carbon double bond using NADH + H+:
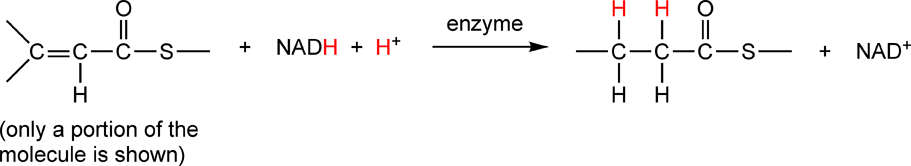
In this reaction, two of the carbons on the left-hand side gain covalent bonds to hydrogen, therefore they are reduced. The NADH + H+ loses covalent bonds to hydrogen, therefore it is oxidized. This reaction is catalyzed by an enzyme; in biochemistry, nearly every reaction is catalyzed by an enzyme. Raymond writes the reaction equation for this reaction in a slightly different way:
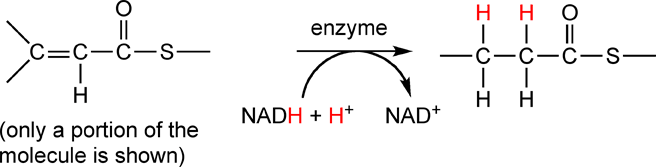
The curved reaction arrow is another way of showing addition reactants and products in a reaction.