The Elaboration-Alkane Structure described how, for organic molecules, more than one molecule can share the same molecular formula, Each of these molecules has a unique structure, and consequently, a unique set of physical and chemical properties. For non-organic molecules we learned how to name molecules based on their molecular formulas. For example, N2O5, is called dinitrogen pentaoxide. On the other hand, calling C6H14 hexacarbon tetradecahydride does not work, because as we saw in Elaboration-Alkane Structure, there are five different molecule with five different structural formulas that share this molecular formula. The 3-dimensional structures for these five molecules are shown below along with their condensed structural formulas:
|
|
|
|
|
The 3-dimensional models for the five constitutional isomers that share the molecular formula C6H14. The condensed structural formulas are shown below each model. Click and drag on each model to view it from different angles. |
||
|
|
 |
To come up with unique names for each of these unique molecules, the names need to be based on the structural formulas instead of the molecular formula. There is a set of rules which are used to create these names called the IUPAC rules. IUPAC is an acronym for International Union of Pure and Applied Chemistry, who are a group of chemists who have been charged with making up rules. The rules as applied for naming alkanes are described in Section 4.4 of Raymond. Below, these rules are applied to come up with name for the five molecules shown above.
|
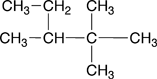
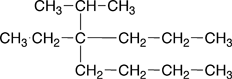
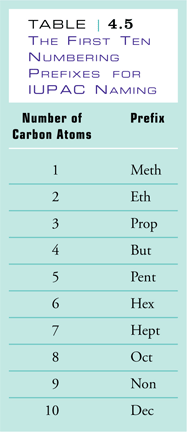
1. Name the parent chain. The name of the parent chain contains two parts. The ending indicates which family the molecule belongs to; in the case of alkanes this ending is -ane. The other part is a prefix, which indicates the number of carbons found in the longest straight chain of carbons in the molecule. These prefixes are shown Table 4.5 of Raymond. The blue boxes drawn around each of the structural formulas below indicates longest straight of carbons for each |
|||
|
hexane |
There are 6 carbons in the longest chain |
 |
|
|
pentane |
There are 5 carbons in the longest chain |
||
|
pentane |
There are 5 carbons in the longest chain |
||
|
butane |
There are 4 carbons in the longest chain |
||
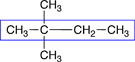 |
butane |
There are 4 carbons in the longest chain |
|
|
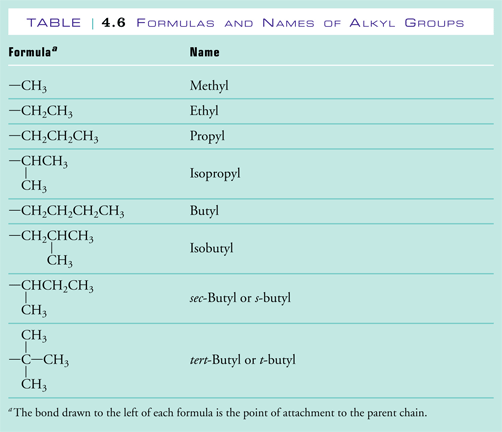
2. Name the alkyl groups. Any group of atoms that is not included in the longest chain of carbon atoms is called a substituent group. When these substituent groups are derived from alkanes they are called alkyl groups. In naming the alkyl groups the -ane ending is replaced by an -yl ending. If the alkyl group is branched, additional prefixes, such as iso-, sec- and tert-, are added to indicate the type of branching. The names of the alkyl groups containing up to 4 carbons are shown in Table 4.6 of Raymond. Each of the alkyl groups in these structures is circled in red and labeled in the figures below. |
|||
|
There are no alkyl groups on this molecule |
|
||
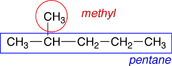 |
There is 1 methyl group on this molecule. |
||
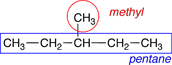 |
There is 1 methyl group on this molecule. |
||
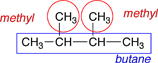 |
There are 2 methyl groups on this molecule. |
||
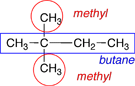 |
There are 2 methyl groups on this molecule. |
||
|
3. Determine the point of attachment of alkyl groups to the parent chain. The longest chain that was used to derive the parent name is numbered starting from the end that is nearest to the first substituent. |
|
|
There are no alkyl groups on this molecule so there is no need to number the parent chain. |
|
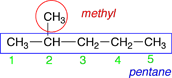 |
The numbering is started at the left-hand end because that end is nearest the methyl group substituent. |
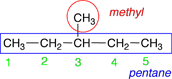 |
For this molecule it does not make a difference which end you start numbering from. |
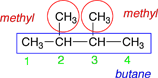 |
For this molecule it does not make a difference which end you start numbering from. |
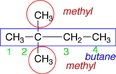 |
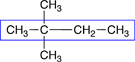
The numbering is started at the left-hand end because that end is nearest the two methyl group substituents. |
|
4. Construct the name of the alkane by placing the names for the alkyl groups in alphabetical order and specifying their position numbers, followed by the name of the parent chain. The labels di, tri, tetra, etc., are added if two or more identical substituents are present. The position numbers are placed in front of the substituent name and separated by a dash. Commas are used to separate multiple substituent numbers. |
||
|
hexane |
The optional prefix n- can be placed in front to indicate that this is normal hexane |
|
 |
2-methylpentane |
The names for the substituents and the parent chain are usually combined into one word. |
 |
3-methylpentane |
|
 |
2,3-dimethylbutane |
Since both substituents are the same, the prefix di- is used to indicate that there are two of them. |
 |
2,2-dimethylbutane |
When there are multiple substituents with the same name, it is important to give a position number for each, even if it is the same number. |