Chemistry and the Black and White Photographic Process: The Making of a Negative
Tim Johnson
H. Baines writes in The Science of Photography that the first camera was in use as early as 400 years ago. These primitive instruments consisted of a large room that projected the inverted image of a still object onto a flat wall. It was called the camera obscura and it was used as an aid to sketching. Of course it was not long after the invention of the camera obscura that attempts were made to render images exact, permanent and portable, but early attempts were unsuccessful. In the early 19th century, Thomas Wedgwood and Sir Humphry Davy used materials such as leather and paper impregnated with silver salts, specifically silver nitrate (AgNO3), to make shadow images of feathers and leaves. But they were unable to prevent the images from darkening further when examined in the light (Baines, pp.12-4).
By 1802, Henry Fox Talbot, now known as the father of modern photography, was making photographs with a camera obscura and paper that was soaked in “common salt solution and then in silver nitrate solution, thus forming silver chloride in the body of the paper. The dried paper required an hour’s exposure before it was sufficiently darkened, but by repeating the impregnation of the paper in this order several times, and exposing it wet, he was able to reduce the exposure time to ten minutes.” Initially Talbot used potassium iodide and sodium chloride to fix his photographs so as to stop them from darkening under further exposure to light. But in 1839, he began using sodium thiosulfate (Na2S2O3), now known to photographers as hypo (Baines, pp.14-7).
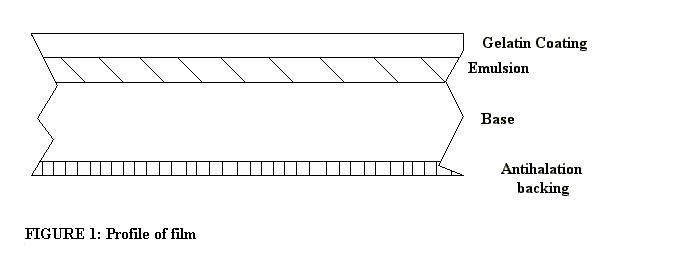
In Photographic Process, Earl N. Mitchell divides modern film into the following components: a gelatin coating, a layer of emulsion, an organic base, and an antihalation backing (see Figure 1).

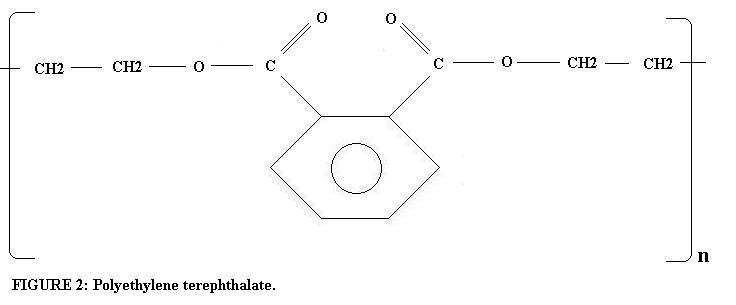
The gelatin coating serves to protect the emulsion, which is prone to scratching. The emulsion is the light sensitive material that is broken down initially by light to form a latent image, and then developed to make the negative. The base is the substrate that the emulsion is applied to and which is rolled and placed into a light tight film canister. The most commonly used base is the organic compound polyethylene terephthalate (see Figure 2).
The antihalation backing serves the important function of absorbing light that goes through all three proceeding layers. Without the antihalation layer, any point source lighting within the frame would appear with a halo surrounding it (Mitchell, pp. 177-8).
The most important of these components to basic photographic principals is the photo emulsion. Modern photo emulsions are made up of three main ingrediants. The first is a combination of silver halide salts (silver bromide, silver chloride and silver iodide) which give film its sensitivity to light. These compounds form lattice work crystals known to photographers as grains(as shown in Figure 3).
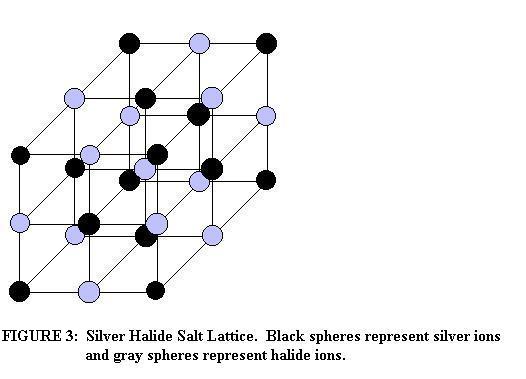
The chemical and physical properties of silver bromide (AgBr), silver chloride (AgCl) and silver iodide (AgI) are quite similar, but the amount of energy required to break each of them apart is sufficient enough to play a role in the property of a given photographic emulsion (Baines, p. 88-9). John and Field report in A Textbook of Photographic Chemistry the minimum amount of light energy required to break apart the different silver halides. Silver chloride is broken down by visible violet and ultra-violet light (light with a short wavelength and relatively high energy), silver chloride is broken down by visible blue light (light rays of relatively lower energy) and silver iodide is broken down by visible green light (the lowest amount of energy required out of the three). By changing the amount of each present, it is possible to change the properties of a given film. For example, an emulsion without silver iodine will not respond to visible light of low energy. It is important to note that waves of higher energy than visible light will break apart silver halide salts also (p.12).
Although it may appear that the main way of controlling the sensitivity of a film is by modifying the amounts of each silver halide present in the emulsion, that is actually not the case. Mitchell points out that “the sensitivity of film is determined primarily by the size of grains in the emulsion,” and “if the grains in an emulsion are all the same size, the film tends to be of high contrast (p. 178).” Baines adds that the smaller the grains, the higher the contrast, but also, the slower the film. Subsequently, it is difficult to make fast, high-contrast film and slow, low-contrast film (p. 97).
The second main component of modern photo emulsions is gelatin. Gelatin is the name given to the clear, gel-like substance made of “starches, gums or glues” that is used to prevent the silver halide salts from precipitating out of solution, keeping them in perpetual suspension. H. Baines explains the importance of gelatin in the production of film:
[The gelatin suspension] can be stored, handled, and coated with little or no settling out of the grains; it can in fact be treated almost as though it were a homogenous liquid. [This nearly homogenous solution] of minute silver halide crystals in gelatin [is] called a photographic emulsion. 91
The last component of photo emulsions is a compound that sensitizes the film. Sensitizing agents are used to help form the latent image. It is only because of these substances that photographers are able to take pictures with very fast shutter speeds (John 37-8). Keep in mind that modern cameras take pictures at 1/100, 1/500 and 1/1000 of a second regularly.
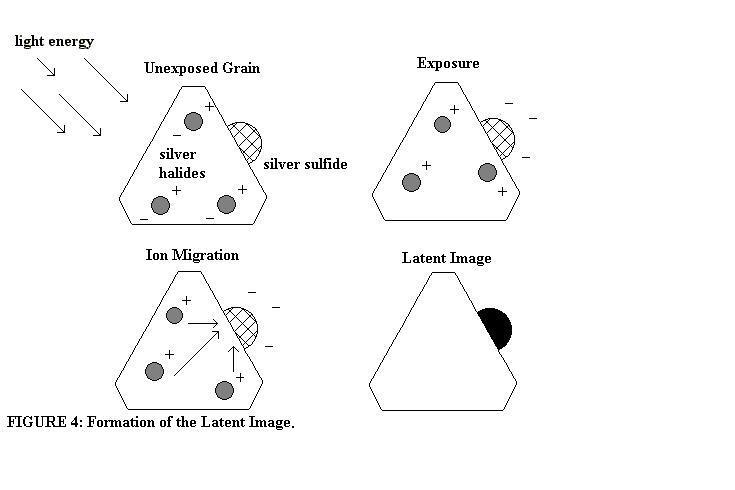
By adding compounds that contain the iso-thiocyanate group (-SCN) during the manufacturing of photo emulsions, silver sulfide (Ag2S) specks can be formed on the surface of silver halide crystals (John, p. 36). When the film is exposed to photons (discrete packets of light energy), the silver halides decompose to form an invisible, yet developable, latent image. Consider silver bromide. When light energy is used to decompose silver bromide, the immediate products are silver ions and bromide ions (AgBr ®Ag+ + Br_). However, with additional energy, bromide ions will release an electron forming elemental bromine atoms (Br_ + energy® Br + e_) (John, p. 6) which then forms bromide molecules (Br2) due to the high reactivity of bromide. The released electrons migrate through the silver halide lattice and are collected by the silver sulfide speck. This charged area of the crystal then attracts interstitial (roaming) positively charged silver ions. The electrons in the silver sulfide “electron trap” then combine with the interstitial silver ions in the area and form metallic silver and a latent image (see Figure 4) (John, pp. 38-9) .
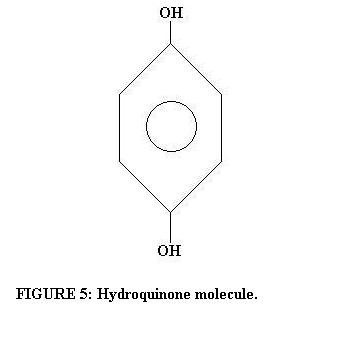
A latent image is virtually undetectable, yet when exposed film is placed into a bath of developer, a useful negative appears. Developers are always reducing agents, meaning they readily donate electrons to substances that will readily accept electrons, and are typically benzene (C6H6) derivative organic compounds. Not just any reducing agent will work however. Satisfactory developing agents quickly convert exposed grains into metallic silver while doing little or no damage to unexposed grains (Baines, p. 123 and p. 126). The typical amount of time needed to develop black and white film is between eight and fourteen minutes. For example, Kodak D-76, a common general-purpose developer, uses hydroquinone (pictured below) as its active reducing agent.
There are still some questions about the exact mechanisms of development, but it is known that the metallic silver speck on the outside of a silver halide crystal acts as a catalyst for the reduction of the entire grain. Thus, as the reaction proceeds and the silver speck increases in size, the reaction rate accelerates on those grains which have been marked by decomposition(Mitchell, pp. 187-8).
The next chemical used in the process of making a black and white negative is stop bath. Developing solution is usually slightly basic. Alkalinity helps to speed up the development process and by neutralizing the developer with a mildly acidic stop bath, the process is slowed and eventually stopped. The most commonly used chemical in an acid stop bath is 3% acetic acid (CH3COOH). There is some debate as to the importance of this step and clean, running water is an acceptable substitute.
At this point in the photographic process, the emulsion contains two components: metallic silver and undeveloped silver halide crystals. The final step in making a black and white negative is removing the undeveloped crystals. If these compounds are left on the negative, when the negative is exposed to light, the unexposed grains will decompose and form silver, thus fogging the entire frame. One of the characteristics of the silver halides is that they are all insoluble in water. The most commonly used fixer, sodium thiosulfate (Na2S2O3), reacts with silver halides to form sodium sulfatoargentate (Na3[Ag(S2O3)2]), a very soluble coordination compound (Baines 147). The reaction steps for the fixing of silver bromide are (Baines, p. 147 and John, p.154:
1) AgBr + 2Na2S2O3 ® Na3[Ag(S2O3)2] + NaBr (formation of Na3[Ag(S2O3)2]) and
2) Na3[Ag(S2O3)2] ®3Na+3 + Ag(S2O3)2-3 (dissociation of Na3[Ag(S2O3)2] in solution)
Now that the unexposed silver halide has been converted into a soluble substance, it is carried away by the fixing solution and a useful negative is left behind.
There is more to the science and process of photography. The negative is then placed under a high intensity lamp and projected onto photo paper. The photo paper is then developed, stopped and fixed. At this point, the intended product has been produced and the process is finished. However, there is much more to the process then is presented here. One can find ample readings on the nature of light and camera lenses, the use of filters in exposure and development, and pushing film, as well as many more chemistry related subjects in the field of photography.
The practicality and usefulness of chemistry is sometimes missed by individuals. For those who enjoy photography or are interested in trying photography, I hope that this is a useful tool in actually understanding the black and white photographic process from exposure to negative.
Works Cited
Baines, H. The Science of Photography. Ed. E.S. Bomback. New York: Halsted, 1976.
John, D.H.O. and G.T.J Field. A Textbook of Photographic Chemistry. London: Chapman & Hall, 1963.
Kodak. “Material Safety Data Sheet.” <www.kodak.com/cgi-bin/webCatalog.pl?cc=US&lc=en&products=KODAK+developer+D-76>, 27 Nov. 2000.
Mitchell, Earl N. Photographic Science. New York: John Wiley and Sons, 1984.