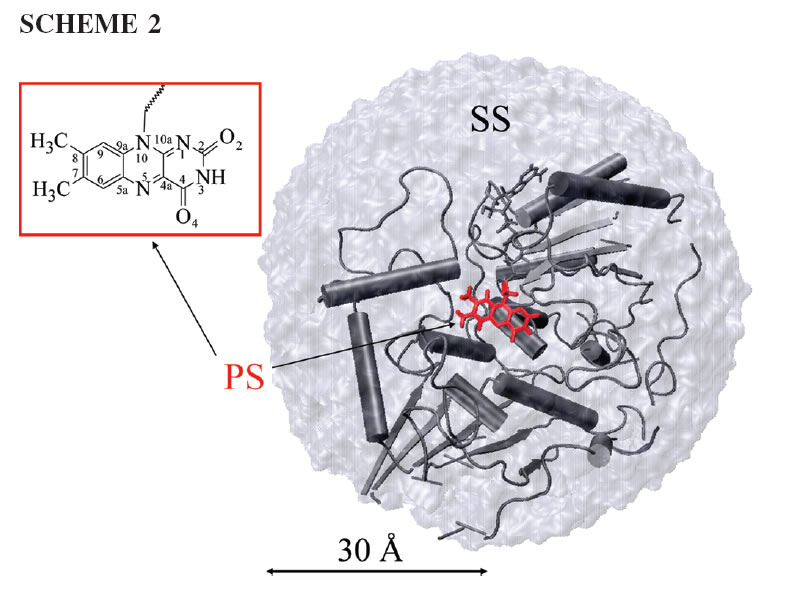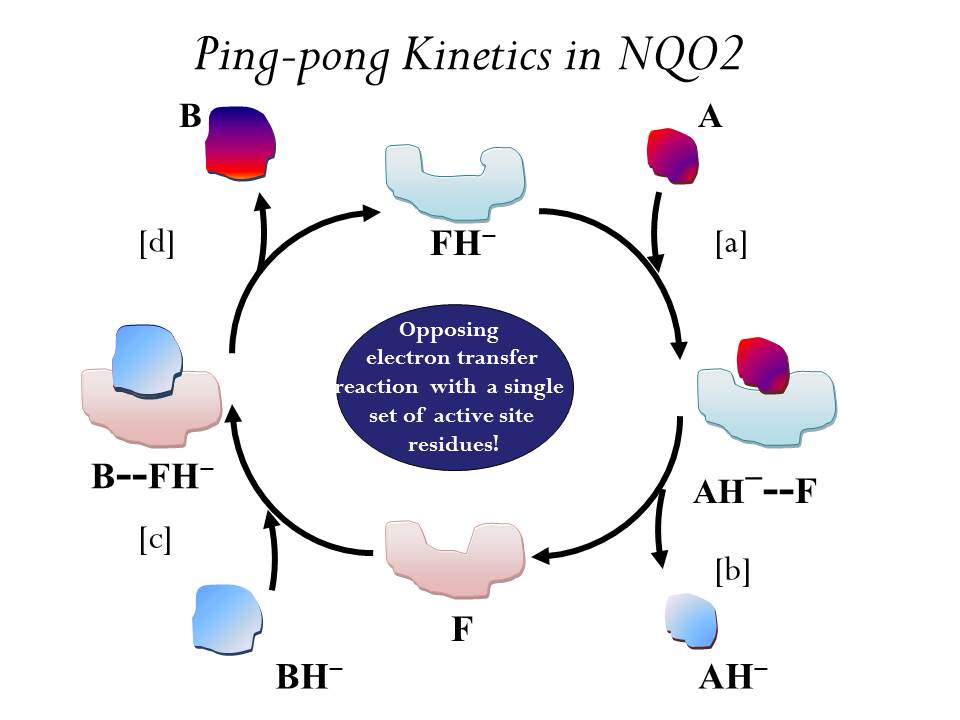
Project 1. Ping-pong Kinetics and Substrate Shuttling in Quinone Oxidoreductase

We are studying the redox chemistry of an enzyme - quinone reductase using theory and molecular simulations. Quinone reductase 2 is detoxifying flavoenzyme and responsible for in vivo conversion of prodrug to anti-cancer drug. The electron transfer occurs through a classic one-site ping-pong mechanism (shown above). In this mechanism the cofactor flavin undergoes cyclic redox reactions and the active site shuttles two substrates -a hydride donor and a hydride acceptor.
Recently, undergraduate researchers Ms. Caitlin Bresnahan, Ms. Clorice Reinhardt, and Thomas Bartholow studied the binding of aromatic molecules with lumiflavin. The thermodynamic quantities for the binding of several polycyclic aromatics were found to be consistent with experimental results. We also found moderate to strong binding for the aromatic groups of protein's own side chains, namely, phenyl alanine, tyrosine, and tryptophan. These results have been published in Journal of Physical Chemistry (doi:10.1021/jp510020v) in 2015.

Earlier, undergraduate researchers Ms. Chee Yang and Mr. Mike North studied a flavin-bound enzyme that exhibited spectacular change in dynamics due to redox changes.These results have been published in Journal of Physical Chemistry in 2011.
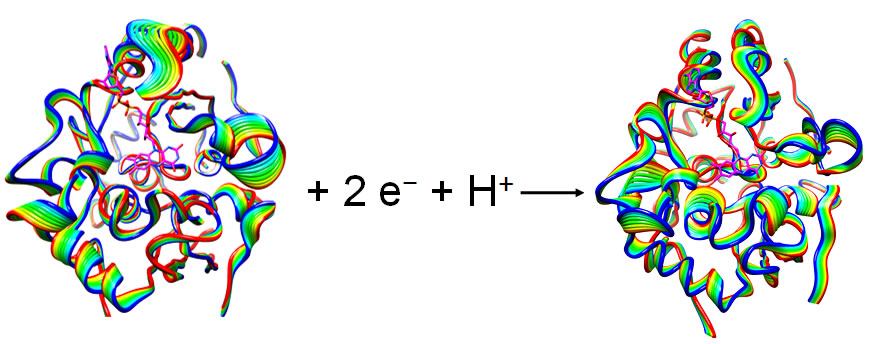
Mr. Mike North, an undergraduate working in the lab, has observed the effect of substitution of flavin ring on the redox process by theoretically computing the redox potentials. This work recently has been published in Journal of Physical Chemistry, in 2010.
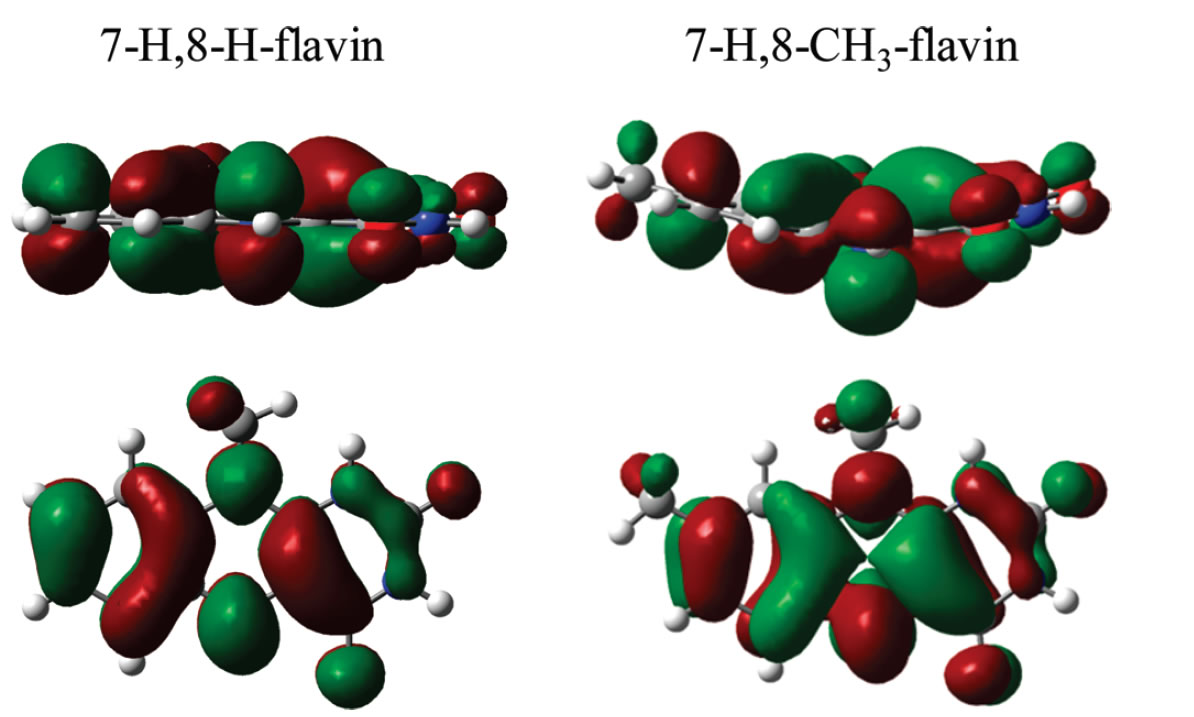
Earlier, undergraduates Mr. James Rauschnot, Ms. Chee Yang, and Mr. Vang Yang have explored the redox behavior of the quinone reductase that activates the anti-cancer drug. We measured the redox potentials of the enzyme using theory and explored the effect of charged residues in the redox process. The work has been published in Journal of Physical Chemistry in 2009.