Project 2. Coupled Domain Dynamics and Communication Pathways in Enzymes (in collaboration with Hati Lab)
We are studying the inter-domain long-range communication in Aminoacyl-tRNA Synthetases (AARSs). Various domains in AARSs, though situated distantly, communicate with each other to carry out the enzymatic function. However, the molecular mechanism of this interdomain coordination in AARSs has remained poorly understood. How do these domains communicate with each other? Does protein dynamics play a role on these long-range communications? We use theory (molecular dynamics simulations) as well as experimental (mutations and kinetic studies)
This year Mr. Alex Strom and Ms. Stephanie Tadayon have performed a detailed study to identify the communication pathway in ProRS. By analyzing the impacts of mutations on dynamics and catalysis, we established the role of these pathways in functional dynamics Biochemistry 2013, 52, 4399-4412).
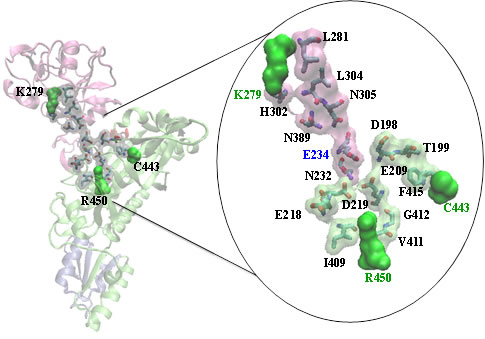
In a recent study, the role of coupled dynamics has been observed in the neighboring structural elements of a catalytic loop in prolyl tRNA synthetase. Mutations performed nearby loop confirms that the coupled dynamics are relevant for catalysis (Sanford et al. Biochemistry 2012, 51, 2146-2156).Three undergraduate students Mr. Bach Cao, Mr. Alex Strom, and Ms. Robyn Mueller have participated in the theroretical part of these studies.

Earlier, undergraduates from our lab we explored the coupled dynamics of structural elements in LeuRS system using bioinformatics and simulations (Weimer et al. J. Biol. Chem. 2009, 284, 10088-99).