Introduction:
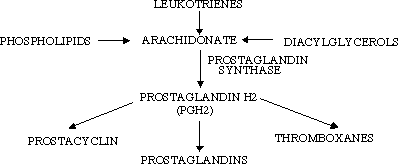
The reaction catalyzed by cyclooxygenase results in the biosynthesis of prostaglandins from their arachadonic acid precursor. Prostaglandins are referred to as local hormones because they are short-lived and only alter the activities of their cell and adjoining cells. This is in contrast to globally active hormones such as insulin. The enzyme responsible for the conversion of arachodonic acid into the prostaglandin precursor is prostaglandin synthase (2).

Prostaglandin synthase is an integral membrane protein found primarily in the smooth endoplasmic reticulum of mammalian tissues (1). Prostaglandin synthase contains two components. The cyclooxygenase component prostaglandin synthase introduces four oxygen atoms onto the intermediate. This step is followed by a two-electron reduction by the hydroperoxidase portion.
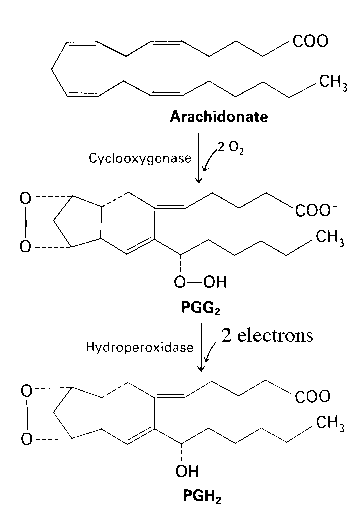
Diagram taken from Stryer, Biochemistry
NSAIDs act upon the cyclooxygenase portion to inhibit the production of prostaglandins. This page will look at the cyclooxygenase/aspirin interaction in depth as aspirin is the only NSAID to covalently modifies cyclooxygenase. Aspirin acetylates a serine residue in the active site of cyclooxygenase.
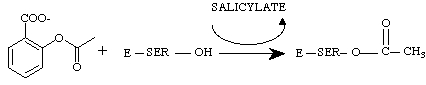
At this point, the enzyme is permanently disabled and prostaglandin synthesis will not resume until more prostaglandin synthase is made (2).