Folic Acid A.K.A. Folate





This site will focus on dihydrofolate reductase because this is the only enzyme for which folic acid is shown as a cofactor in the Protein Data Bank files.
|
Folic Acid A.K.A. Folate
|
||||||||||||||
 |
||||||||||||||
| Folic acid is a carrier of one-carbon fragments which it can transfer to various targets in biochemical reactions. The one-carbon piece can be in several different oxidation states but two import forms of folic acid from a medical point of view are methyl-tetrahydrofalate (methyl-THF) and methylene-THF. The figure below shows some key reactions which involve one-carbon transfers. | ||||||||||||||
 |
||||||||||||||
| Folic acid consists of a pteridine base attached to p-aminobenzoic acid (PABA) and glutamic acid. Animals cannot make p-aminobenzoic acid or attach glutamic acid to aminobenzoic acid, so all of the folic acid which we need is ultimately derived from microbial and plant sources. The structure of folic acid is shown below (after uptake into the cell additional glutamic acid residues may be added to the one already present): | ||||||||||||||
 |
||||||||||||||
| Folic acid is not the active form used in our bodies. Once in our cells, folic acid is reduced twice to produce the active form - tetrahydrofolate. To make this easier to see, only the part which undergoes changes is shown in the figure below. | ||||||||||||||
 |
||||||||||||||
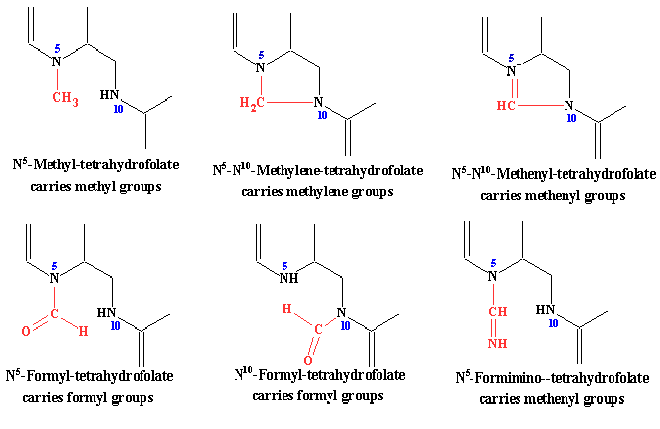
| Tetrahydrofolate is a coenzyme in many reactions involving the transfer of onr carbon units to other compounds. It can carry one carbon groups of all oxidation states, except carbon dioxide. The single carbon groups can be carried on N5, N10 or bridged between both these nitrogens: | ||||||||||||||
 |
||||||||||||||
| One very important reaction which utilizes folate is the synthesis of deoxythymidylate (dTMP), one of the four deoxyribonucleotides required for DNA synthesis. In this reaction N5,N10-methylene tetrahydrofolate transfers a methyl group to deoxyyuridylate (dUMP) to form dTMP as shown below: | ||||||||||||||
 |
||||||||||||||
| Dihydrofolate is reduced back to tetrahydrofolate by folate reductase (see above) and is recharged with a methylene group in a reaction involving serine. However, the main point here is that N5,N10-methylene tetrahydrofolate is absolutely essential for DNA synthesis. This is especially true in cells that are dividing rapidly such as red blood cell producing bone marrow cells, hair follicles, intestinal mucosa cells and cancer cells which are rapidly replicating their DNA. An analogue of folic acid called methotrexate selectively binds to and inhibits dihydrofolate reductase. It bind over1000 times more tightly than folate does, inhibiting the conversion of folate and dihydrofolate into the active form, tetrahydrofolate. Methotrexate is a powerful anti-cancer drug which limits the synthesis of dTMP, which then inhibits DNA replication. | ||||||||||||||
| Another important role of folate in biochemistry is in methylation reactions. The most important carrier of methyl groups is S-adenosyl-methionine (SAM). After SAM gives up its methyl group, it is S-adenosyl-homocysteine which is hydrolyzed to homocysteine. Homocysteine must be methylated to re-form homocysteine. Methyl-THF is the methylating agent in this reaction.
This site will focus on dihydrofolate reductase because this is the only enzyme for which folic acid is shown as a cofactor in the Protein Data Bank files. |
||||||||||||||
Let's take a look at Dihydrofolate Reductase (DHFR) |
||||||||||||||