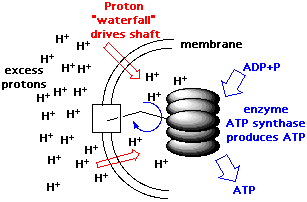
| In Boyer's model, the key to
this process is a tiny shaft running through the middle of a barrel-like
portion of the enzyme. A flow of protons through the membrane makes the
shaft spin, which sucks in raw materials and blows out the fresh ATP. The
model has been instrumental in overturning simplistic "lock-and-key"
explanations of how enzymes work, in which chemicals simply drop into inflexible
enzyme grooves, react and then depart. |
The structure contains 22722 atoms and 23211
bonds connected as 2987 amino acid groups.
These build a 3D protein macro-structure which contains 7 protein chains
:
A ,
B ,
C ,
D ,
E ,
G (this
last one is the barrel link shaft which spins, shown better with this view
).
The protein structure contains 120 helix units and
94 sheet units and
the x-ray structure was found to contain 5 ligated ADP molecules . |