Different acids and bases have different strengths. This means that at a given concentration, different acids and bases produce different hydronium (hydrogen) or hydroxyl ion concentrations when dissolved in water. Strong acids produce the highest concentrations because they completely dissociate (Arrhenius definition) or donate their protons (Brønsted-Lowry definition) 100% of time. Calculating the hydronium ion concentration for a strong acid is rather straight forward; it is just equal to the concentration of the acid times the number of hydrogen ions it releases or donates per molecule. The "Try This" for Part II of the Elaboration - Definitions of Acids and Bases gives an example of how this is done.
Determining the hydronium ion concentrations for strong bases works similarly, but there is an extra step. It is the hydroxyl ion concentration that is equal to the concentration of the the base, times the number of hydroxyl ions it releases per molecule. To determine the hydronium ion concentration, the hydroxyl ion concentration is divided into the Kw value: [H3O+] = Kw/[OH-]. The "Try This" for Part II of the Elaboration - Definitions of Acids and Bases gives an example of how this is done. The number of strong acids and bases is rather small. Table 9.5 in Raymond lists most of them. Most acids and bases, especially those that we we encounter in biological chemistry, are weak acids and bases.
Weak Acids and Bases
Weak acids and weak bases produce a hydronium or hydroxyl ion concentration that is less than their total concentration. We have already seen an example of this when we were discussing ammonia as a Brønsted-Lowry base (see Figure 1 in Part III - Elaboration - Definitions of Acids and Bases). Using the Virtual Lab Simulator we were able to demonstrate how a 0.01 M aqueous solution of ammonia (NH3) produces a pH of 10.6, while earlier, in the "Try This" for Part I of the Elaboration - Definitions of Acids and Bases we saw that a 0.01 M solution of the strong base NaOH produces a pH of 12.0. Clearly aqueous ammonia is a weaker base than sodium hydroxide because it does not increase the pH above 7.0 as much as sodium hydroxide does. Calculating hydronium or hydroxyl ion concentrations produced by weak acids and bases is less straight forward than for strong acids and bases. It involves using the equilibrium constant for the acid or base reaction that produces the hydronium or hydroxyl ion. We will not be doing these calculations in this course, but we will be using the equilibrium constants to compare the relative strengths of different acids and bases.
The Acidity Constant (Ka)
The concepts of chemical equilibria and equilibrium constants is introduced in Section 9.3 of Raymond. The acidity constant (Ka) is the equilibrium constant for the reaction that produces the hydrogen ion (Arrhenius definition) or the hydronium ion (Brønsted-Lowry definition). At equilibrium, the concentrations of the reactants and products in a reaction stop changing. The equilibrium constant is a characteristic for a reaction and is determined by dividing the equilibrium concentrations of the products of the reaction by the equilibrium concentrations of the reactants. Any component of the reaction whose concentration is unaffected by the reaction is left out of the calculation. As an example, we will use the acid/base reaction of acetic acid. Acetic acid is a carboxylic acid and is characteristic of most of the biological acids we will encounter this semester. When added to water (H2O), acetic acid (CH3COOH) donates a proton to water to produce an acetate ion (CH3COO-) and a hydronium ion (H3O+):

The equilibrium constant for this reactions is
![]()
The [H2O] concentration is left out of the denominator, because as the solvent its concentration is not measurably affected by the reaction with the acetic acid. The subscript "a" is used instead of "eq" to denote that this equilibrium constant for an acid/base reaction. The equilibrium constant can be used to compare the relative amounts of product and reactant present at equilibrium. Table 9.2 in Raymond provides a rule-of-thumb way of interpreting equilibrium constants (Figure 1).
 |
In the acid/base reaction for acetic acid, one of the products is the hydronium ion (H3O+). In general, strong acids have Ka values greater than 10 and weak acids have Ka values less than 0.1. To illustrate this, let us use the Virtual Laboratory Simulator to find the equilibrium concentrations of the components present in a 0.01 M solution of acetic acid and use them to determine the acidity constant, Ka, for acetic acid. To do this you can open up the Virtual Laboratory Simulator by clicking here and loading the Unit 6 - Elaboration homework (File > Load Homework > Unit 6 > Elaborations - Definitions of Acids and Bases). If you need to review how to use the Virtual Laboratory Simulator go to Part I of Elaborations - Definitions of Acids and Bases or view the video tutorial. After using the Simulator to make up 100 mL of 0.01 M CH3COOH and using it to measure the pH this solution, you should get something that looks like Figure 2.
 |
First, Figure 2 shows us that 0.01 M CH3COOH has a pH of 3.38, and, therefore, is indeed an acid. This pH value is also greater than the pH of 2.0 we observed for a 0.01 M solution of HCl (see Figure 5, Part I, Elaboration - Definitions of Acids and Bases), meaning acetic acid is less acidic than HCl. The Virtual Laboratory Simulator also shows us the molar concentrations of the various components in solution at equilibrium:

As we can see, 0.0004097 moles/liter of the initial 0.02 moles/liter of the the CH3COOH have donated a proton to water to produce 0.0004097 moles/liter of CH3COO- and 0.0004097 moles/liter of H3O+. The 0.0004097 moles/liter of H3O+ produced caused the pH to drop to 3.38:

At equilibrium, we also see that only a small percentage of the the acetic acid (CH3COOH) has donated its proton to water.


This is the nature of weak acids, unlike strong acids, which react to form nearly 100% product, weak acids react to only produce a small amount of product, 4% in the case of acetic acid. However, this small amount of product is able to reduce the pH appreciably; in this case from 7.00 to 3.38
The Virtual Laboratory Simulator also provided us with enough data to calculate the acidity constant, Ka, for acetic acid:

As expected, this value is much less than 1, confirming the characterization of acetic acid as weak acid. As with hydrogen ion concentrations, there is also a "p" scale that is used with Ka values. The pKa is defined as -log(Ka). Our determined value for the pKa of acetic acid is then

The higher the pKa, for an acid, the weaker the acid. Figure 3 shows Table 9.4 from Raymond. It lists a group of acids, arranged according to acid strength. The acids at the top of the list are the strongest and have the highest Ka values and the corresponding lowest pKa values, while the acids at the bottom of the list are the weakest and have the lowest Ka values and the corresponding highest pKa values.
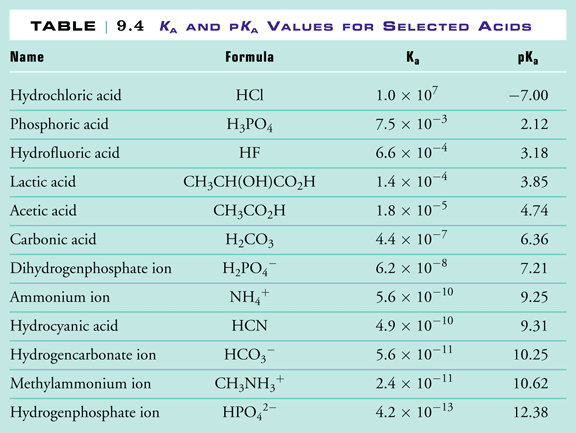 |
Note that our calculated values for Ka and pKa based on the data we obtained in our virtual laboratory agree with the textbook values shown in Figure 3.