Renal Calculi
Susan
Meyer
Honor's
Chemistry 115
Dr.
Fred King
(University
of Wisconsin Eau Claire)
November
29, 2000
Renal Calculi
Introduction.
Over one million Americans each year are admitted to hospitals for the treatment of nephrolithiasis, or renal stone disease. And this is not just a recent phenomenon. Many people throughout history, including Benjamin Franklin and Isaac Newton, suffered from kidney stones. With this condition, hard masses of crystal deposits build up inside the kidney, growing to sizes anywhere from microscopic to an astounding 3 pounds. In humans these “stones” can potentially form in anyone, but are most prevalent in white males between 20 and 30 years old. Renal Calculi, (kidney stones) sometimes cause infection or obstruction in the urinary pathway, but the main issue is simply the extreme pain of passing the stone out of the kidney and through the ureter. (See figure 1.) About 90% of stones formed do pass on their own. Others need to be removed by some sort of medical intervention. Stones are not a common cause of renal failure or death, but are merely painful and bothersome.
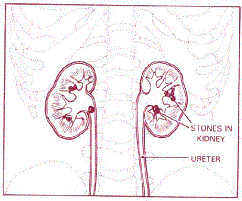
Figure 1. Renal Calculi in Human Kidney
In this project, emphasis will be on the formation and composition of different types of stones. An overview of the diagnosis and specific treatment of nephrolithiases will also be included.
I.
Precipitation of Supersaturated Substances in Urine
Example: Calcium Oxalate
Calcium oxalate crystals are the most common crystal found in kidney stones. Needed for bone formation and other key body processes, calcium ions are abundant in the body. C2O4-, the oxalate ion comes from disassociated oxalic acid, which is produced in the body and also taken in through foods like broccoli, rhubarb, oranges, coffee, and various other sources. Since the body can’t break it down any further, C2O4- must be eliminated through the urine. (Urine is formed in the kidneys.) Dissolved calcium and oxalate ions can combine to form calcium oxalate, which can then precipitate to solid form if the concentration of aqueous calcium oxalate becomes great enough. These are the two equilibrium equations taking place:
Ca2+(aq) + C2O4-(aq) ßà CaC2O4(aq)
CaC2O4(aq) ßà CaC2O4(s)
CaC2O4 can only precipitate if the urine is supersaturated with this solute. Supersaturated means that its concentration exceeds its solubility. In other words if there is too much calcium oxalate for it to all remain completely dissolved, some will precipitate out of solution. So solubility is important.
One factor that affects solubility is pH. Low urine pH, or highly acidic urine, increases the solubility of calcium oxalate. This is because oxalate anions react with hydrogen ions, reducing the concentration of oxalate ions available to react with the calcium cations in the urine. This causes the direction of the first equilibrium equation (above) to shift left in order to compensate for the depletion and maintain the equilibrium constant, (Le Chatelier’s Principle). The concentration of CaC2O4 (aq) is therefore lessened, reducing the likelihood that precipitation will occur. (Note: For other types of stones, low acidity can work in the opposite way to actually promote stone formation.)
Other factors also restrain the concentrations of stone-forming substances. Urine citrate, an example of the natural stone inhibitors present in urine, also plays an important role. Urine citrate can form a soluble salt with calcium ions, lowering the concentration of Ca+ ions in solution. This prevents stones in the same way as the hydrogen ions in the pH example above—by reducing available reactants. If the level of urine citrate is too low, calcium oxalate stones are more likely to appear.
Even if urine does become supersaturated, with CaC2O4 for example, precipitation does not necessarily occur. Most people’s urine is in fact supersaturated with calcium oxalate, but obviously not all people form stones. In order for precipitation to start, the concentration must exceed what is called the ‘super saturation limit’, to become ‘labile’. It then can undergo the first step of precipitation—nucleation.
Nuclei are the tiniest particles of precipitate that can form and grow in a supersaturated solution. Molecules unite and form clusters in a series of reversible, unkinetically favored reactions. If the urine is only slightly over its ‘super saturation limit’, nucleation is very slow and a precipitate takes a long time to appear. The process is speeded by the presence of other submicroscopic particles in urine. CaC2O4 can cling to the surface of another crystal and begin the formation of calcium oxalate calculi.
The second step in precipitation is spontaneous crystal growth. The nucleic ‘clusters’ continue to grow into larger and larger clumps of crystals until the concentration of the aqueous calcium oxalate is no longer higher than its solubility. In other words, stones continue to grow until the urine is no longer supersaturated with the precipitating substance. The rate of crystal growth is directly related to how concentrated the reactants are. The more supersaturated the urine is the faster the stones grow.
II. Composition and Causes of Stones
Stones are not composed entirely of one type of crystal. During precipitation other substances are often carried down with the precipitate. This is called co precipitation and accounts for the variety and impurities of stones. Over 200 different components have been found in analyzed stones, but the stones are classified by their major component. This table shows the four basic types of stones and what is in them.
TYPE OF STONE |
MAJOR COMPONENT |
|
Calcium Oxalate |
CaC2O4 |
|
Uric Acid |
Crystallized Uric Acid |
|
Struvite |
Mg, PO4, NH4 |
|
Cystine |
Cystine (an amino acid or protein monomer) |
Table 1. Basic Types of Renal Calculi and Their Components
Calcium oxalate stones are usually caused by hypercalciuria or hyperoxaluria. This are basically just overproduction or over absorption of calcium or oxalate in the body. This happens as a result of abnormalities in metabolic processes and can be hereditary. Thiazide diuretics and small changes in diet can effectively prevent more stones from forming.
Uric acid stones occur in highly acidic urine or because of massive over excretion of urinary uric acid. It’s initially treated by alkanizing the urine with bicarbonate or citrate. Eating less meat and fish can help reduce uric acid levels. A medication called allopurinol also fights against high uric acid concentration in urine which leads to stone formation.
Struvite stones are called ‘infection stones’. A certain type of bacteria that usually invade the kidney after a person has been taking antibiotics for something causes them. The best prevention against struvite stones is to exercise caution in overuse of antibiotics. Recent research has shown that acetohydroxamic acid can slow the formation of this type of stone.
Cystine stones are very rare. They are caused by a genetic defect that is manifested in cystinuria, a protein transfer disorder in the kidneys. There are no real treatments for this condition. Patients are supposed to drink an enormous amount of water all the time to reduce the urinary cystine concentration.
III. Diagnosis and Removal of Renal Calculi
Diagnosing a person with kidney stones is fairly straightforward. Bloody urine is one sign that stones are present, somewhere in the urinary tract. The most attention-getting symptom, however, is the extreme pain that arises, characteristically starting in the lower back/abdominal region and moving down toward the pelvis. Again, this is caused from the stone passing, or attempting to pass, out of the kidney and down the ureter. The actual passage of a stone out of the body (through the urethra) is another very conclusive sign of nephrolithiasis. If a stone has formed it will usually be followed by the formation of more stones consecutively, unless conditions of the urine are changed.
Slightly more complicated than the general diagnosis of renal calculi is determining the source of the problem. As discussed previously, stones form from a variety of different disorders or substance imbalances in the urine. In order to know what treatment to provide, doctors must take measures to find out what type of stones are precipitating. Blood chemical profiles and urine analyses can give clues by showing any abnormal levels of substances like Ca2+, citrate, or uric acid in solution. These also show any abnormal pH levels, which could also give insight as to likely stone type.
Stones themselves can be analyzed to determine what kind of crystals they contain. Several techniques are used including polarization microscopy, x-ray crystallography, and even infrared analysis. (One company, the L. C. Herring & Co. Laboratory, that analyzes thousands of body calculi annually, have some great pictures of different kidney stones up on their website: http://www.herringlab.com/photos/.) Once the type of stone has been determined the source of trouble is more easily pinned down and treatment can be decided upon.
Only a small percentage of stones need to be medically removed since most pass on their own. If a stone is causing severe blockage it must be dealt with. Sometimes stones are surgically extracted. Other techniques, such as cytoscopic removal and coagulum pylolithomy, are used in certain cases. Another very interesting treatment for removing small stones is called ESWL, or extra corporeal shock wave lithotripsy. As its name suggests, this technique uses shock waves to actually break up kidney stones so they can pass through the ureter.
Conclusion
The formation of renal calculi in the kidneys affects many people. It is a very clear example of simple chemical phenomena in the body. In order to understand why stones occur, and how to better treat and prevent them, it is necessary to grasp the chemical principles behind their appearance. (i.e. solubility and acidity)
SOURCES:
Burns, D.T., Townshend, A., Catchpole, A. G., Inorganic Reaction Chemistry: Systematic Chemical Separation, Ellis Horwood Limited West Sussex, England, 1980.
Brown, Claire, Analyses of the In Vivo Crystallization of Calcium Oxalate, online.
http://www.keele.ac.uk/depts/ch/groups/csg/cltb/clairehome.htm
NIH Consensus Statement, Prevention and Treatment of Kidney Stones, mar28-30; 7(1): 1-23, 1988 online. http://odp.od.nih.gov/consensus/cons/067/067_statement.htm
NIH Publication No. 83-2495, August 1987.